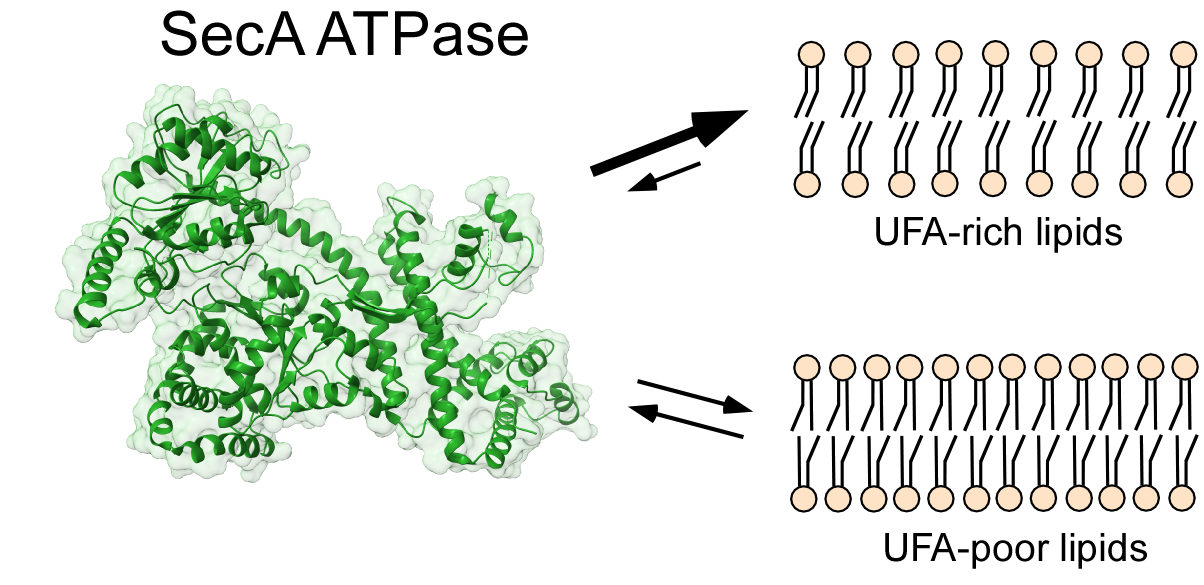
The translocon SecYEG and the associated ATPase SecA form the primary protein secretion system in the cytoplasmic membrane of bacteria. The secretion is essentially dependent on the surrounding lipids, but the mechanistic understanding of their role in SecA:SecYEG activity is sparse. Here, we reveal that the unsaturated fatty acids (UFAs) of the membrane phospholipids, including tetraoleoyl-cardiolipin, stimulate SecA:SecYEG-mediated protein translocation up to ten-fold. Biophysical analysis and molecular dynamics simulations (done by the team of Prof. Dr. Holger Gohlke) show that UFAs determine the loose packing of lipid head groups, where the N-terminal amphipathic helix of SecA docks. UFAs promote SecA binding to the membrane, and SecA:lipid interactions convert into the augmented translocation. Our results identify the fatty acid structure as a notable factor of SecA:SecYEG activity, which may be crucial for protein secretion in bacteria, which actively change their membrane composition in response to their habitat.
See the publication here